Referring Patients
to the FUEL-2 Trial
About the FUEL-2 Study
The FUEL-2 Study is a Phase 3, double-blind, placebo-controlled study researching the efficacy and safety of an investigational medication, udenafil, for teenagers 12–18 years of age who had the Fontan procedure.
We are currently recruiting participants. We would like to ask for your help by referring any patients who may be eligible, with their permission. Please feel free to discuss any information about the FUEL-2 Study with your patient.
References
Karamlou T, et al. J Thorac Cardiovasc Surg. 2018;155:1727-1731.
Lopez KN, et al. Am Heart J. 2022;11:e025358.
Soskolne G, et al. Pediatr Crit Care Med. 2012;13:218-219
Diversity among our clinical study participants is important, as racial and ethnic minorities are often not well represented in clinical studies (1-3)
Although life expectancy for patients with Fontan physiology has improved, disparities in morbidity and mortality remain due to systemic inequities. (2)
African and Hispanic populations, in particular, tend to undergo the Fontan procedure later in life, and have
limited access to long-term care. (3)We request your help in considering referral of patients who reflect the population of people with Fontan physiology.

Why is the FUEL-2 study being conducted?
Children born with single ventricle heart disease (SVHD) require multiple surgeries for their long-term survival, including the Fontan procedure (1).
After the Fontan procedure, pulmonary vascular resistance becomes critical for effective vascular circulation (2).
Although well-tolerated in childhood, Fontan physiology deteriorates over time, giving rise to cardiac and noncardiac morbidities.
Individuals who have undergone the Fontan procedure experience limitations in their exercise capacity. These limitations reflect this abnormal physiology and are predictors of cardiovascular morbidity and mortality (3).
Udenafil, a selective phosphodiesterase type 5 (PDE5) inhibitor, was shown to be safe and effective in improving exercise capacity in adolescents with Fontan physiology (4).
However, further studies are needed to support these findings.
Clinical Evidence Supporting FUEL-2
A new post-hoc analysis (2025) of the original FUEL trial demonstrates that adolescents with Fontan circulation and baseline peak VO₂ <80% predicted experienced significant improvements in peak VO₂, ventilatory anaerobic threshold metrics, and myocardial performance with udenafil therapy (5). These findings support the results from FUEL and helped to inform the study design for the FUEL-2 protocol.
Read the complete analysis
References
Maxey TS, et al. Critical Heart Disease in Infants and Children. 3rd ed. Philadelphia, PA: Elsevier; 2019. pp. 747-757.e2
Egbe AC, et al. Circ Heart Fail. 2017;10:1-8.
Dennis M, et al. J Am Coll Cardiol. 2018;71:1009-1017.
Goldberg DJ, et al. Circulation. 2020;141:641-651
Goldberg DJ, et al. J Am Heart Assoc. 2025; 125.041348
Study Overview
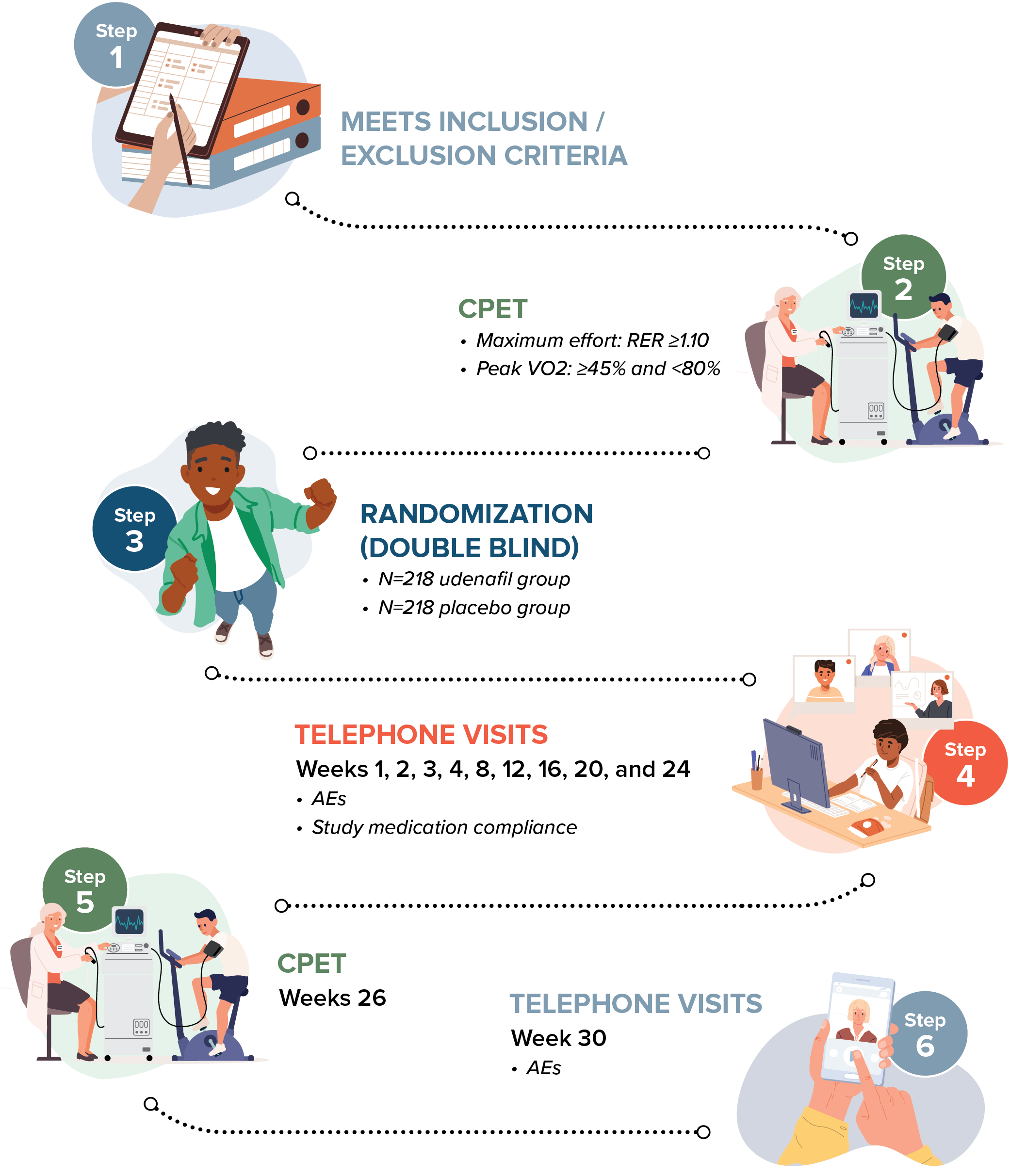
The FUEL-2 Study will include up to 436 teenagers and their participation could last for approximately 7 months.
The study is divided into 3 parts:
Screening visit: 1 day
Study treatment period: 6 months
Follow-up period: 1 month
The study has a brief visit schedule, involving 2 study visits to the study center, and up to 11 phone calls.
Eligible participants will be randomized 1:1 to receive either udenafil or placebo, twice a day, for about 26 weeks.
Eligibility Criteria
Key Inclusion Criteria
12–18 years of age, inclusive, with Fontan physiology.
Current antiplatelet or anticoagulant therapy.
Key Exclusion Criteria
Height <132 cm.
Peak VO2 <45% or ≥80% of that predicted for age and gender.
Use of PDE5 inhibitors within 12 months or other
pulmonary hypertension medication within 3 months.Hospitalization for acute decompensated heart failure within the last 12 months.
Undergoing evaluation or listed for heart transplantation.
Study Endpoints
Primary Efficacy Endpoint
Change in peak minute oxygen consumption (VO2, mL/kg/min) from Baseline to Week 26, as measured by maximal cardiopulmonary exercise testing (CPET).
Secondary Efficacy Endpoint
Changes from Baseline to Week 26 in:
VO2 (mL/kg/min) at the ventilatory anaerobic threshold (VAT)
Enhanced liver fibrosis (ELF) score
Work rate (watts) at VAT
Ventilatory efficiency (VE/VCO2) at VAT.
Primary Safety Assessments
Safety will be assessed by monitoring treatment-emergent adverse events (TEAEs).
Heart rate, systolic/diastolic blood pressure, and cardiac rhythm will be assessed continuously during CPET.
Study Design
Current Clincial Trial Sites
Eligible participants may be compensated for their time. All travel-related costs (airfare, lodging, mileage, and related expenses) will be fully covered.
Store locator is loading from StoreRocket Store Locator App..